Introduction
Severe cutaneous adverse reactions (SCARs) — such as DRESS, AGEP, and SJS/TEN — are among the most serious drug hypersensitivity syndromes.
The traditional approach is simple: avoid all suspected drugs indefinitely.
But this blanket avoidance often creates real-world problems:
- Patients lose access to first-line therapies (e.g., anti-tuberculosis drugs, antiepileptics, antibiotics).
- Treatment becomes suboptimal or unsafe when alternatives are inferior.
- Many patients are left with multiple “allergy labels”, even though usually only 1–2 drugs were true culprits.
This raises an important question:
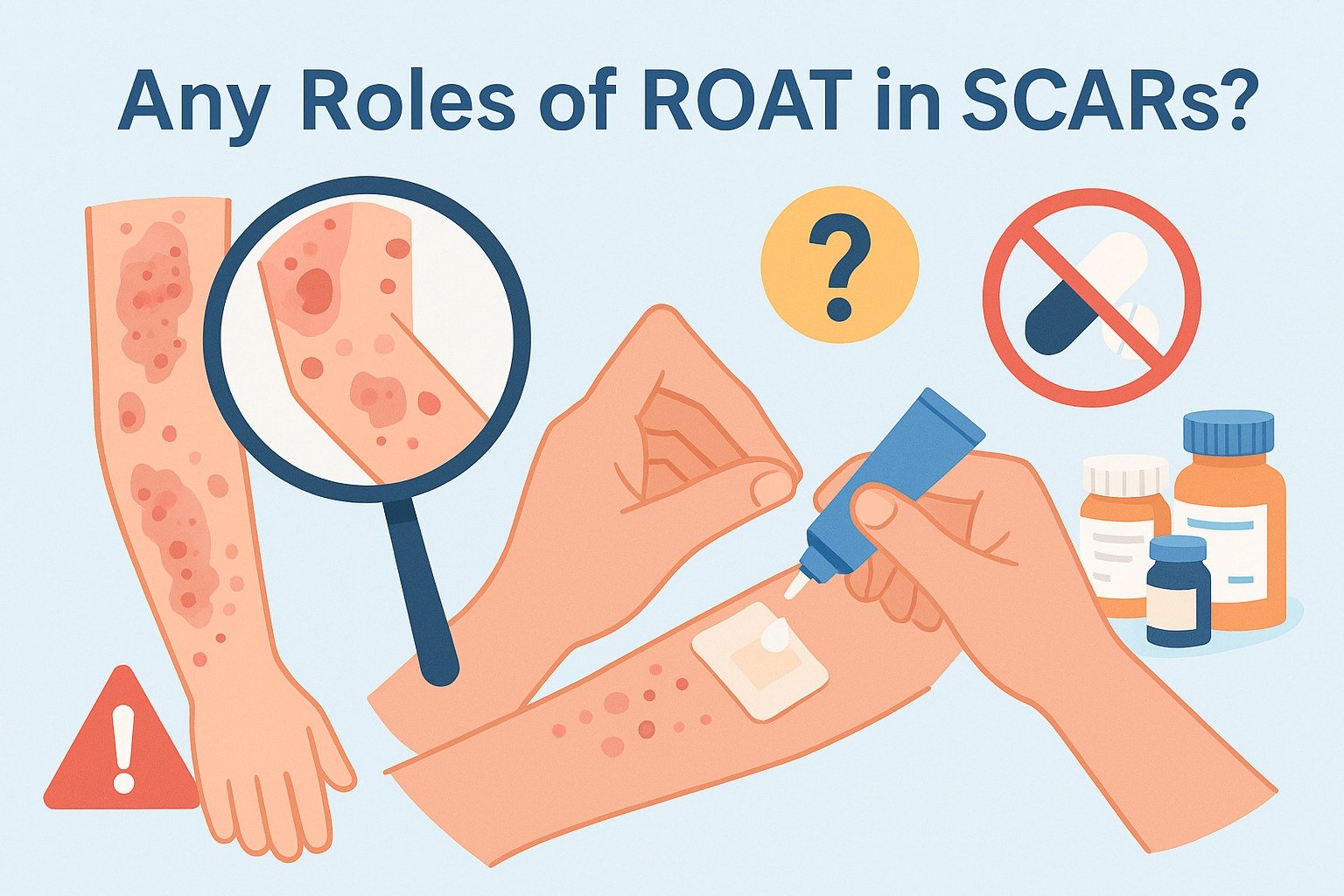
👉 Is it time to reconsider the role of patch testing — and even the Repeated Open Application Test (ROAT) — in managing SCARs?
Why ROAT Is Feared in SCARs
The Repeated Open Application Test (ROAT) is a simple diagnostic tool in delayed hypersensitivity:
- A diluted preparation of the suspected drug is applied on the skin (usually inner forearm) twice daily for several days.
- It is widely used in contact dermatitis and topical drug reactions.
Concerns in SCARs:
- Risk of systemic reactivation (e.g., DRESS relapse).
- Progression to life-threatening recurrence (e.g., SJS/TEN).
- Lack of large prospective safety data.
⚠️ Yet, despite these fears, no fatalities from ROAT or patch testing in SCAR patients have been reported to date.
The Cost of Over-Avoidance
While caution protects patients, over-avoidance has consequences:
- Loss of effective treatment → rifampin for TB, carbamazepine for epilepsy.
- Increased morbidity from weaker second-line drugs.
- Permanent “drug allergy labels” without diagnostic confirmation.
💡 In TB/HIV-endemic countries, clinicians sometimes perform carefully supervised drug re-introductions (with steroid cover if needed) because avoiding first-line agents is more dangerous than controlled re-exposure.
Real-World Data: Challenging the Dogma
1. Patch testing in SCARs
- Australian studies show patch testing in DRESS/AGEP patients can be performed safely in expert centers.
- In FDE, in-situ patch tests and ROAT are safely used to confirm culprits.
2. Drug challenges under necessity
- South African cohorts: patients with anti-TB drug–induced DRESS were rechallenged when no alternatives existed, with reactions controlled by early corticosteroids.
3. ROAT observations
- Case reports describe topical antibiotics triggering localized but controlled pustules in AGEP.
- FDE patients often tolerate and benefit from ROAT to identify the offending drug.
Risk Stratification: When ROAT Might Be Feasible
| Reaction Type | ROAT Feasibility | Rationale |
| AGEP | ✅ Consider | Usually drug-specific; localized testing rarely systemic. |
| FDE (not a SCAR) | ✅ Recommended | Confined to fixed sites; multicenter data support safety. |
| DRESS | ⚠️ With caution | Possible systemic reactivation; only for essential drugs under monitoring. |
| SJS/TEN | ❌ Contraindicated | High mortality and systemic relapse risk. Use in vitro tests instead. |
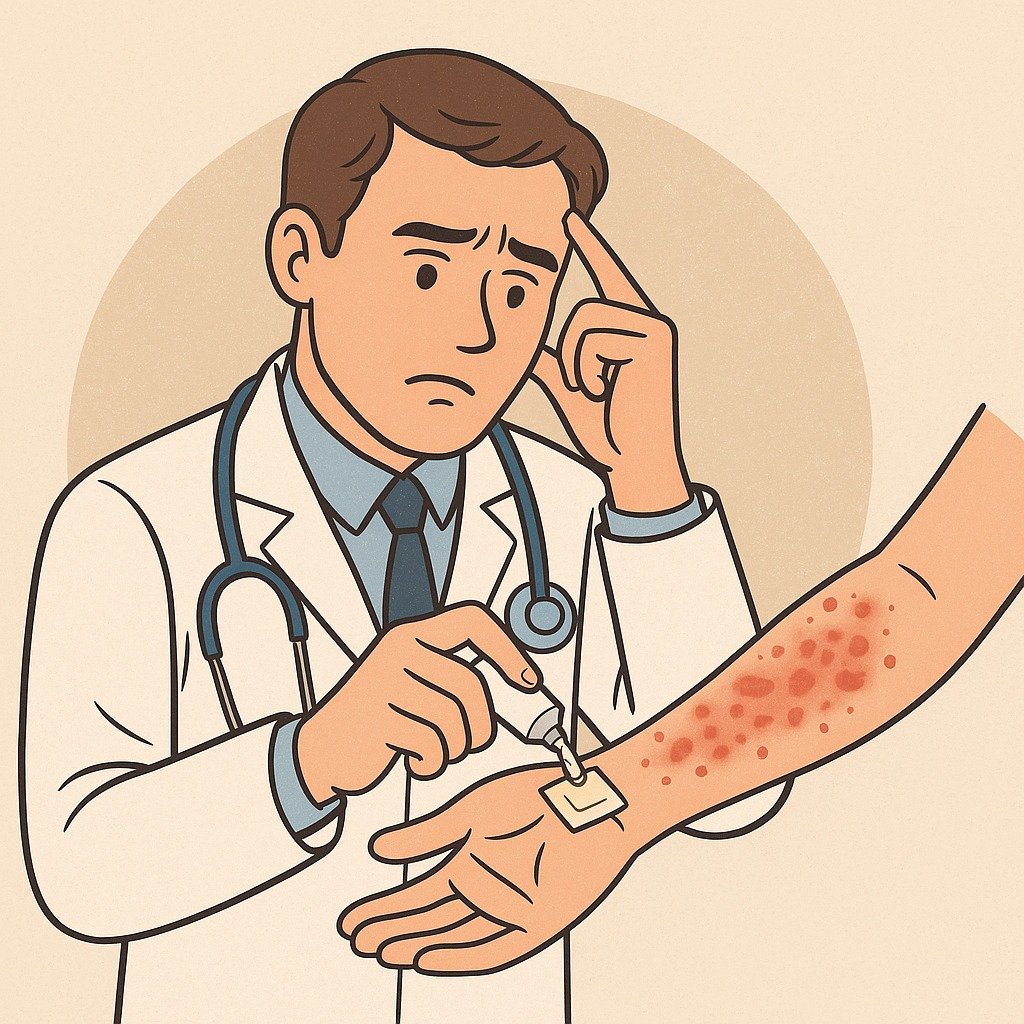
A Structured ROAT Protocol (If Considered)
Step 1: Patient Selection
- Exclude SJS/TEN.
- Wait ≥6 months post-SCAR.
- Prioritize essential or irreplaceable drugs.
Step 2: Test Execution
- Preparation: 1% concentration of suspected drug in petrolatum.
- Application: Inner forearm, pea-sized, twice daily × 7 days.
- Monitoring: Daily skin review; labs for DRESS patients.
- Stop criteria: Spreading erythema, fever, systemic symptoms.
Step 3: Post-Test Management
- Negative: Consider graded oral challenge if drug is essential.
- Positive: Confirm culprit, document for lifelong avoidance.
Ethical & Practical Considerations
- Informed consent: Explain risks, off-label status, lack of guideline endorsement.
- Medicolegal protection: Only in specialist centers with emergency backup.
- Alternatives: Patch testing, lymphocyte transformation test (LTT), HLA genotyping.
🟡 Key Takeaways
- ROAT is safe and recommended in FDE, with multicenter evidence supporting its role.
- AGEP may tolerate ROAT under specialist care.
- DRESS is a gray zone — possible only when essential drugs are irreplaceable.
- SJS/TEN: ROAT should not be attempted.
- Over-avoidance can be as harmful as the reaction itself. Individualized, supervised strategies matter most.
Conclusion
The future of SCAR management lies in precision, not dogma.
While ROAT remains off-limits in SJS/TEN, emerging data suggest it may have a role in FDE, AGEP, and carefully selected DRESS cases when alternatives are scarce.
👉 That said, if ROAT continues to be viewed with concern in SCARs, the path forward should be formal clinical trials. A logical first step would be to study its safety and utility in not-so-severe SCARs such as AGEP and mild DRESS before considering wider use.
References
- The combined utility of ex vivo IFN-γ release enzyme-linked immunospot assay and in vivo skin testing in patients with antibiotic-associated severe cutaneous adverse reactions. The Journal of Allergy and Clinical Immunology: In Practice. 2018 Jul 1;6(4):1287-96.
- A multicentre study to determine the value and safety of drug patch tests for the three main classes of severe cutaneous adverse drug reactions. British Journal of Dermatology. 2013 Mar 1;168(3):555-62.
- In situ patch test and repeated open application test for fixed drug eruption: a multicenter study. The Journal of Allergy and Clinical Immunology: In Practice. 2024 Feb 1;12(2):460-8.
- First-line antituberculosis drug challenge reactions in drug reaction with eosinophilia and systemic symptoms syndrome in an HIV endemic setting. The Journal of Allergy and Clinical Immunology: In Practice. 2024 Oct 1;12(10):2798-808.
- Early high-dose intravenous corticosteroids rapidly arrest Stevens Johnson syndrome and drug reaction with eosinophilia and systemic symptoms recurrence on drug re-exposure. The Journal of Allergy and Clinical Immunology: In Practice. 2021 Jan 1;9(1):582-4.
Key message:
ROAT should not be dismissed outright — but before being embraced in SCARs, it deserves rigorous clinical study to ensure safety and guide practice.
This article reflects current evidence limitations and should not be interpreted as clinical practice recommendations. Any consideration of ROAT in SCAR patients should involve specialized centers with appropriate expertise and safety protocols.