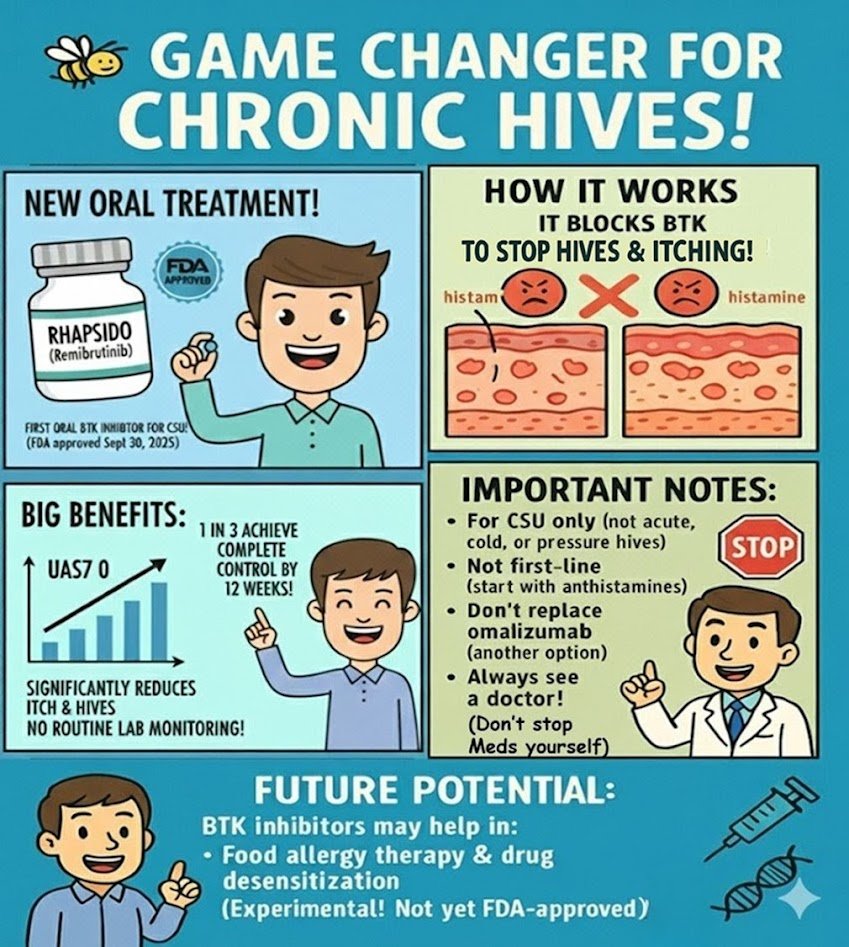
For years, patients with chronic spontaneous urticaria (CSU) who didn’t respond to antihistamines had only injections as the next step. On September 30, 2025, the U.S. Food and Drug Administration (FDA) approved remibrutinib (brand name Rhapsido), the first oral BTK inhibitor for adults with CSU. This approval offers patients — and doctors — the first convenient oral option beyond antihistamines. What Is Chronic Spontaneous Urticaria (CSU)? Why an Oral Option Matters Until now, patients had only these alternatives: Patients have long asked for a safe oral treatment that avoids injections…
Read MoreWednesday, October 15, 2025
Recent posts
- Game Changer: FDA Approves Rhapsido (Remibrutinib), the First Oral BTK Inhibitor for Chronic Hives (CSU)
- Drug vs Food Allergy: Immediate & Delayed Reactions
- Sulfa Allergy: Safe Drugs, Drugs to Avoid, and How to Think About Cross-Reactivity
- ROAT Patch Testing in SCARs: Is It Time to Reconsider?
- FDA Warns of Severe Rebound Itching After Stopping Cetirizine (Zyrtec) or Levocetirizine (Xyzal): What You Need to Know
Your Guide to Drug Allergy Mastery