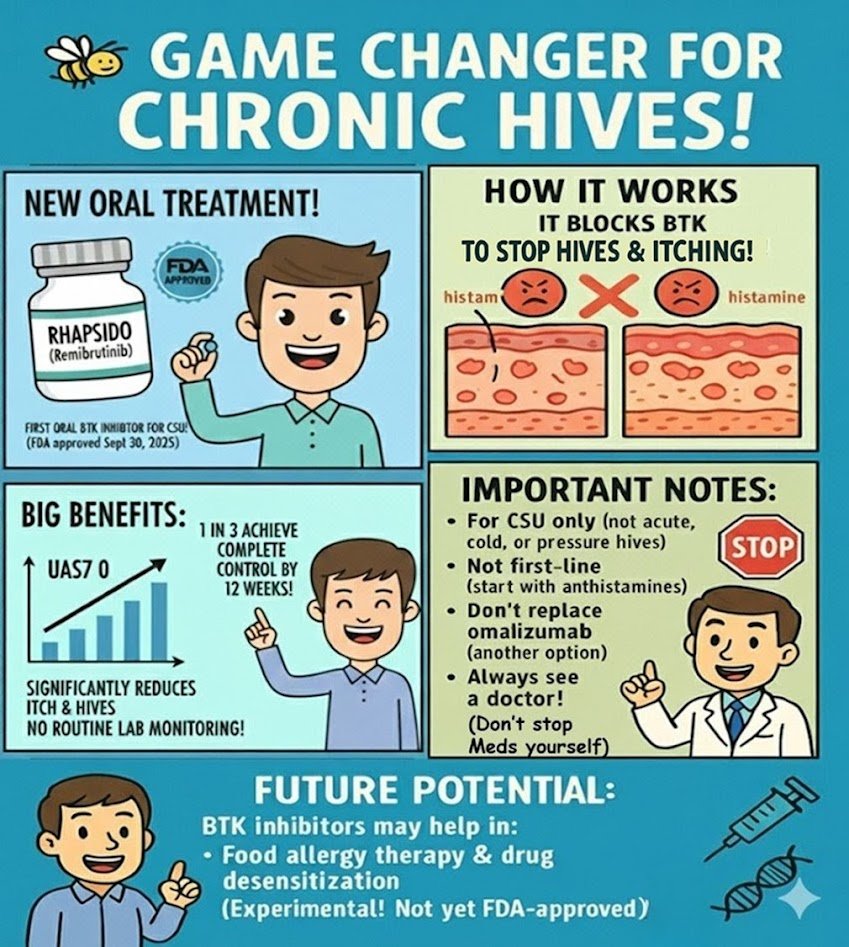
For years, patients with chronic spontaneous urticaria (CSU) who didn’t respond to antihistamines had only injections as the next step. On September 30, 2025, the U.S. Food and Drug Administration (FDA) approved remibrutinib (brand name Rhapsido), the first oral BTK inhibitor for adults with CSU.
This approval offers patients — and doctors — the first convenient oral option beyond antihistamines.
What Is Chronic Spontaneous Urticaria (CSU)?
- Daily hives and itching for more than 6 weeks without a clear trigger
- Often includes angioedema (swelling)
- Symptoms disrupt sleep, work, and quality of life
- First-line treatment: H₁-antihistamines, often at higher doses
- Challenge: Up to 50% of patients remain symptomatic, creating an unmet need
Why an Oral Option Matters
Until now, patients had only these alternatives:
- Omalizumab (Xolair®) — an injectable anti-IgE antibody, effective but requires injections every 2–4 weeks
- Cyclosporine — sometimes used off-label, but with safety concerns and frequent monitoring
Patients have long asked for a safe oral treatment that avoids injections and heavy monitoring.
Rhapsido: A BTK Breakthrough
| Feature | Details |
| Class | Bruton’s Tyrosine Kinase (BTK) inhibitor |
| How it works | Blocks BTK, calming mast cells and basophils that release histamine |
| Dose | 25 mg twice daily (oral tablet) |
| Efficacy | In Phase III REMIX-1 & REMIX-2, many patients improved within 12 weeks; ~1 in 3 had complete control (UAS7 = 0) |
| Safety | No routine lab monitoring required; more favorable than cyclosporine |
How It Compares
| Treatment | Route | Notes |
| H₁-antihistamines | Oral | First-line; limited efficacy in many |
| Omalizumab | Injection | Proven effective but requires injections |
| Cyclosporine | Oral | Off-label, requires close monitoring |
| Remibrutinib (Rhapsido) | Oral | First oral targeted CSU therapy; no monitoring needed |
Pitfalls Patients Should Know
- ❌ Not for all hives — only for CSU, not acute or inducible types (cold, pressure, etc.)
- ❌ Not first-line — antihistamines remain the foundation
- ⚠️ Omalizumab still important — Rhapsido is an alternative, not a replacement
- ⚠️ Long-term safety — clinical trials are promising, but real-world data are still limited
- 💲 Access & cost — currently FDA-approved only in the U.S.; not yet available elsewhere
Beyond Hives: What’s Next for BTK Inhibitors?
Research suggests BTK inhibitors could play a role in:
- Food allergy immunotherapy — early trials showed improved peanut tolerance when combined with OIT
- Drug desensitization (chemo & biologics) — BTK inhibition may reduce severe reactions in high-risk protocols
These uses are experimental and not FDA-approved, but they highlight the broader potential of BTK inhibitors.
The Drug Detective’s Final Word
The FDA approval of Rhapsido (remibrutinib) is a landmark milestone for CSU treatment. It’s not a cure-all, but it provides a long-awaited oral option for patients who remain uncontrolled on antihistamines.
Looking ahead, BTK inhibitors may reshape food allergy and drug desensitization strategies — if future trials confirm their promise.
📌 For patients: Don’t stop or switch medications on your own. If you think Rhapsido might be right for you, talk with your allergist or dermatologist to see how it fits into your treatment plan.
🔑 Key Takeaways
- ✅ First oral targeted therapy for CSU: FDA approved Sept 30, 2025
- 💊 Blocks BTK to reduce mast cell & basophil activity
- 📈 Trials: ~1 in 3 patients achieved complete control of hives by 12 weeks
- ⚠️ Approved only for CSU unresponsive to antihistamines
- 🧪 Future potential in food allergy & desensitization (not FDA-approved)
- 👨⚕️ Patients should discuss with their allergist before considering this option
📦 Patient Summary
The FDA has approved remibrutinib (Rhapsido) — the first pill for people with chronic spontaneous hives (CSU) who don’t improve with antihistamines.
- Many patients saw fewer hives and less itching; some became hive-free.
- It’s not for all hives and not the first treatment doctors try.
- Omalizumab (injection) and other options are still important.
- Always talk to your doctor before making treatment changes.
📚 References
- Kaul M, et al. Clin Transl Sci. 2021;14(5):1756–68.
- Suresh RV, et al. J Clin Invest. 2023;133(16):e168793.
- Giavina-Bianchi P, et al. J Allergy Clin Immunol. 2024;153(4):1075–85.
- Rodsaward P, et al. J Allergy Clin Immunol Pract. 2023;11(2):642–4.
- Novartis. Novartis receives FDA approval for Rhapsido (remibrutinib), the only oral targeted BTKi treatment for chronic spontaneous urticaria (CSU) [Internet]. Basel: Novartis; 2025 Sep 30 [cited 2025 Oct 5].