AGEP vs GPP: When Skin Reactions Look Similar but Behave Very Differently Skin rashes can be alarming, especially when they appear suddenly and spread quickly. Two conditions that often cause confusion are acute generalized exanthematous pustulosis (AGEP) and generalized pustular psoriasis (GPP). AGEP vs GPP is a comparison that often raises confusion among both patients and clinicians. These two skin conditions can appear strikingly similar at first glance, yet their causes, clinical behavior, and long-term management are very different. Understanding these differences is important — not only for accurate diagnosis,…
Read MoreAuthor: drugallergydetective.com
Adult-Onset Immunodeficiency From Anti–Interferon-Gamma Autoantibodies: The Underrecognized Cause of Opportunistic Infections in HIV-Negative Adults
This condition—often called Adult-Onset Immunodeficiency Syndrome (AOIDS)—is one of the most important but overlooked causes of severe opportunistic infections in HIV-negative adults with normal CD4 counts. Awareness remains low, especially outside Asia, despite an increasing number of global cases. 🔎 Quick Summary: When to Suspect Adult-Onset Immunodeficiency Adult-onset immunodeficiency due to anti–interferon-gamma (anti–IFN-γ) autoantibodies should be considered in HIV-negative adults—especially those with Asian ancestry—who develop unexplained, recurrent, or disseminated intracellular infections despite normal lymphocyte counts. Key clinical clues Pneumocystis jirovecii pneumonia (PJP) can occur but is significantly less common than…
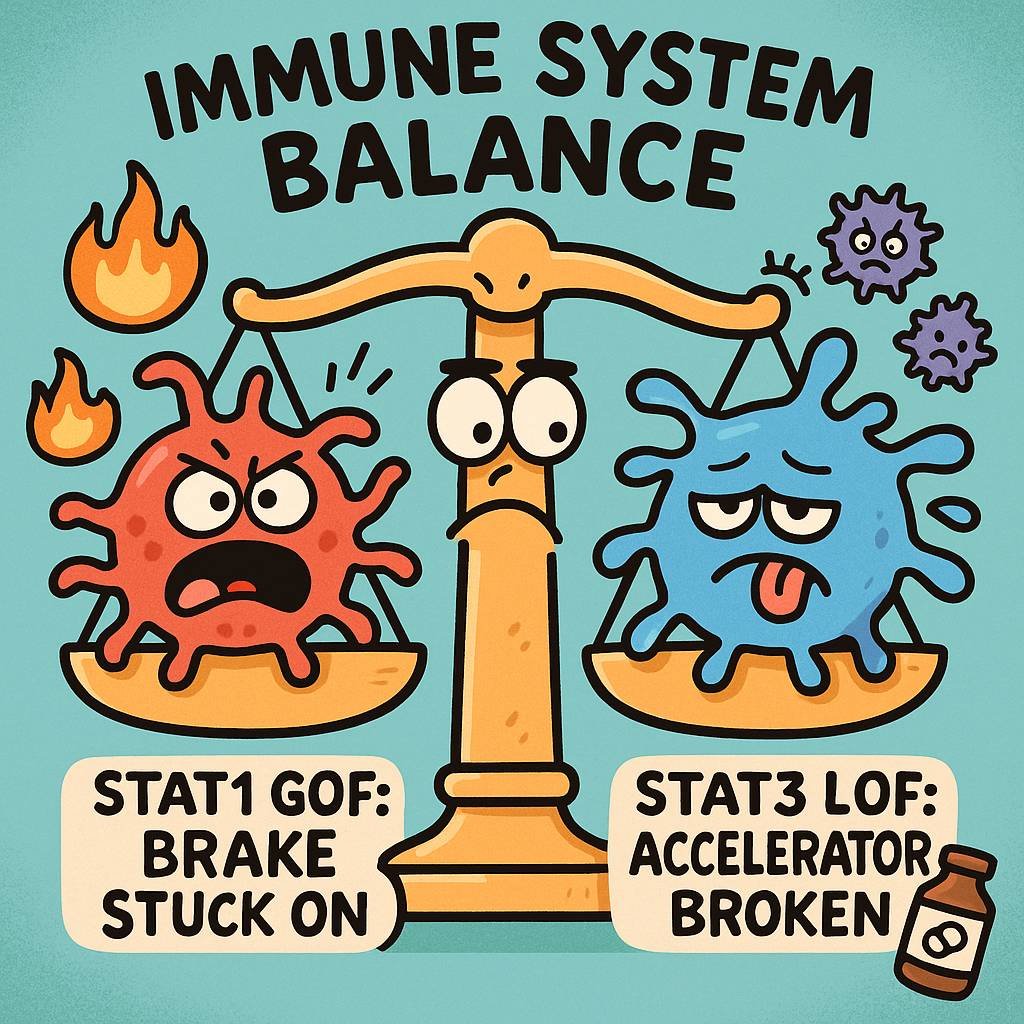
Read More🧠 STAT1 Gain-of-Function vs. STAT3 Loss-of-Function: Understanding Opposite Immune Dysregulation
Educational Content Disclaimer This article provides educational information about rare immunodeficiency disorders. It is not medical advice and should not replace consultation with qualified healthcare providers. Individual presentations vary, and management should be personalized under medical supervision. 🔗 Why This Matters for Drug Allergy Detective At Drug Allergy Detective, we focus on helping you understand drug allergies and hypersensitivity reactions. But to truly understand why certain people react to medications differently, we need to explore the immune system’s foundation. This article explores two rare genetic conditions that represent opposite extremes…
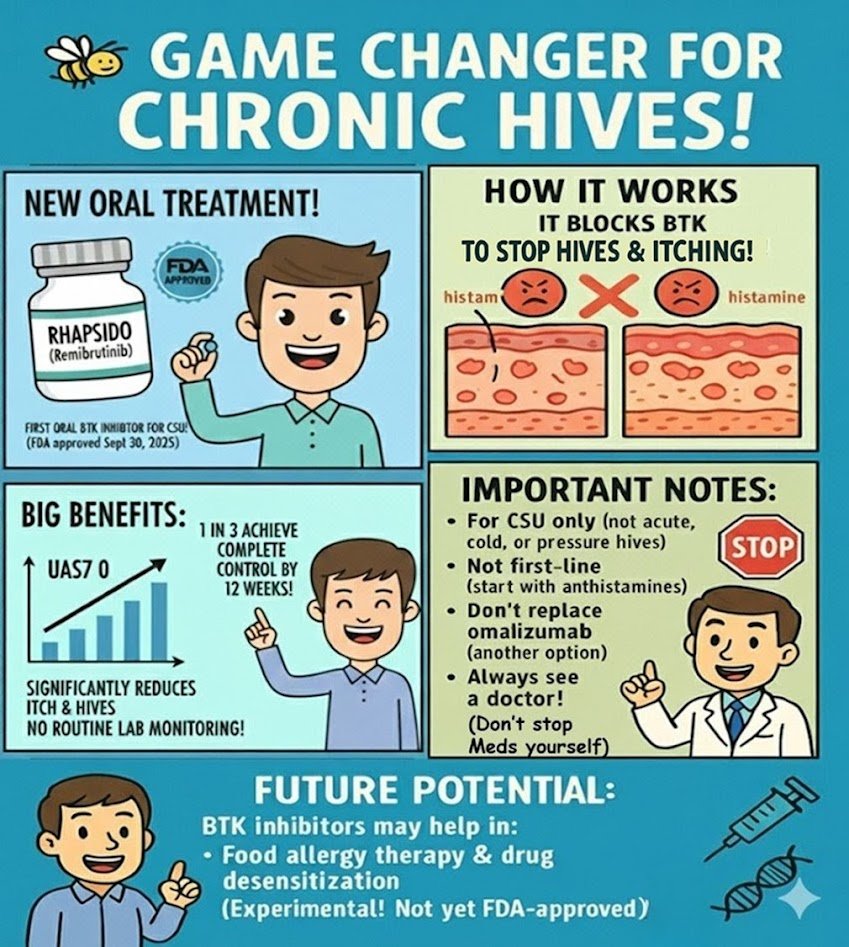
Read MoreGame Changer: FDA Approves Rhapsido (Remibrutinib), the First Oral BTK Inhibitor for Chronic Hives (CSU)
For years, patients with chronic spontaneous urticaria (CSU) who didn’t respond to antihistamines had only injections as the next step. On September 30, 2025, the U.S. Food and Drug Administration (FDA) approved remibrutinib (brand name Rhapsido), the first oral BTK inhibitor for adults with CSU. This approval offers patients — and doctors — the first convenient oral option beyond antihistamines. What Is Chronic Spontaneous Urticaria (CSU)? Why an Oral Option Matters Until now, patients had only these alternatives: Patients have long asked for a safe oral treatment that avoids injections…
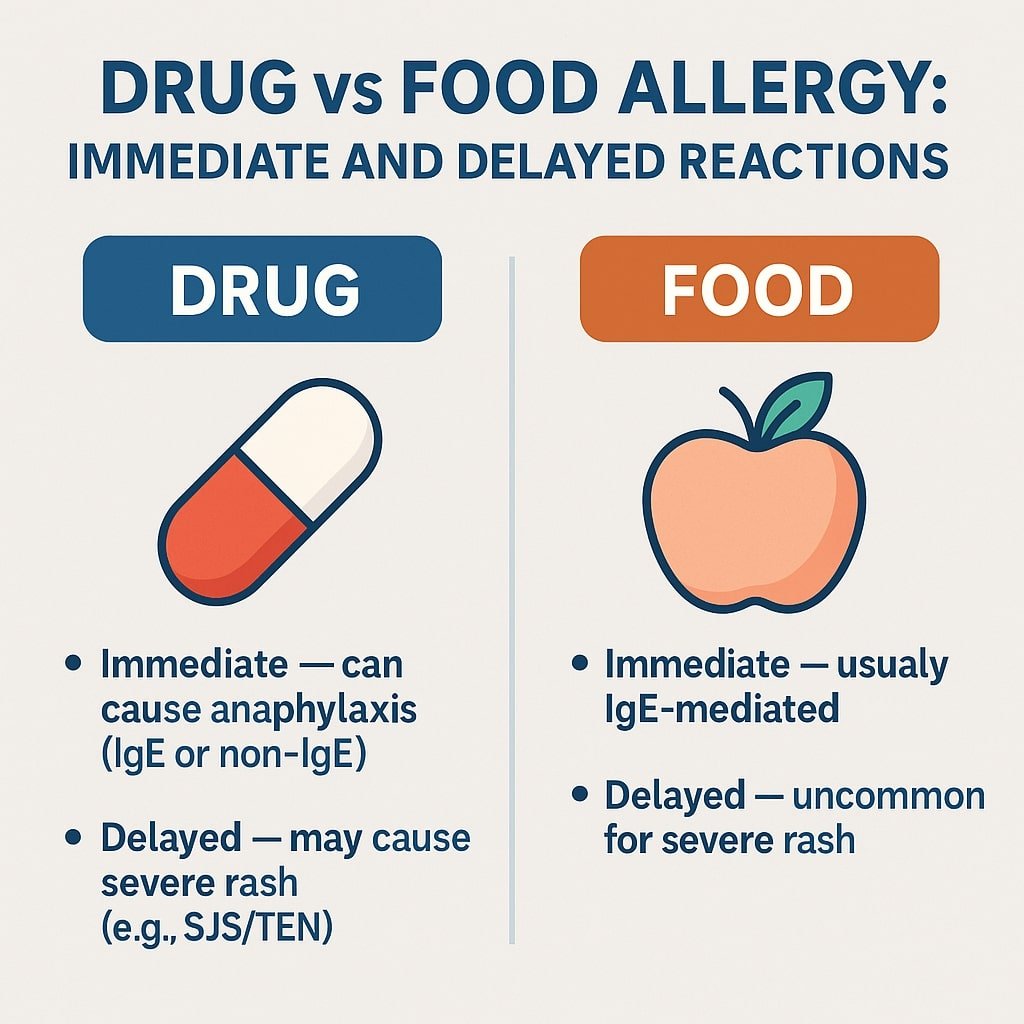
Read MoreDrug vs Food Allergy: Immediate & Delayed Reactions
Drug vs Food Allergy: Why Medications Can Cause Both Immediate and Delayed Reactions — While Foods Uncommonly Cause Severe Delayed Reactions This article is for educational purposes only. Always consult healthcare providers for personalized medical advice. Patients often ask:“Why can medications cause both rapid allergic reactions like hives or anaphylaxis and dangerous delayed reactions such as Stevens–Johnson Syndrome (SJS) or Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS), while food allergies almost always appear immediately?” The difference lies in how the immune system processes drugs versus foods — and in…
Read MoreSulfa Allergy: Safe Drugs, Drugs to Avoid, and How to Think About Cross-Reactivity
Many patients search online for sulfa allergy safe drugs after experiencing severe reactions to antibiotics such as co-trimoxazole (trimethoprim-sulfamethoxazole). When people hear the term “sulfa allergy,” confusion often follows. Some patients wonder if they must avoid every drug with “sulfa” in the name. The truth is: not all sulfa-containing medications are dangerous. The key is understanding the chemical structure—specifically whether the drug contains the N4 arylamine group. The Real Culprit: The N4 Arylamine Group The allergy risk in sulfonamide antibiotics comes from a specific chemical feature known as the N4…
Read MoreROAT Patch Testing in SCARs: Is It Time to Reconsider?
Introduction Severe cutaneous adverse reactions (SCARs) — such as DRESS, AGEP, and SJS/TEN — are among the most serious drug hypersensitivity syndromes.The traditional approach is simple: avoid all suspected drugs indefinitely. But this blanket avoidance often creates real-world problems: This raises an important question:👉 Is it time to reconsider the role of patch testing — and even the Repeated Open Application Test (ROAT) — in managing SCARs? Why ROAT Is Feared in SCARs The Repeated Open Application Test (ROAT) is a simple diagnostic tool in delayed hypersensitivity: Concerns in SCARs:…
Read MoreFDA Warns of Severe Rebound Itching After Stopping Cetirizine (Zyrtec) or Levocetirizine (Xyzal): What You Need to Know
🛑 New FDA Safety Alert: Antihistamine Withdrawal Can Trigger Severe Itching In May 2025, the U.S. Food and Drug Administration (FDA) issued a new safety warning about rebound itching after stopping cetirizine (Zyrtec) or levocetirizine (Xyzal)—two of the most commonly used antihistamines. This severe itching reaction, often mistaken for allergy relapse, may occur especially in people who have taken the drug daily for several months. 🔎 Key Findings from the FDA Investigation Between April 2017 and July 2023, the FDA identified 209 confirmed cases globally—197 in the U.S.—involving moderate to…
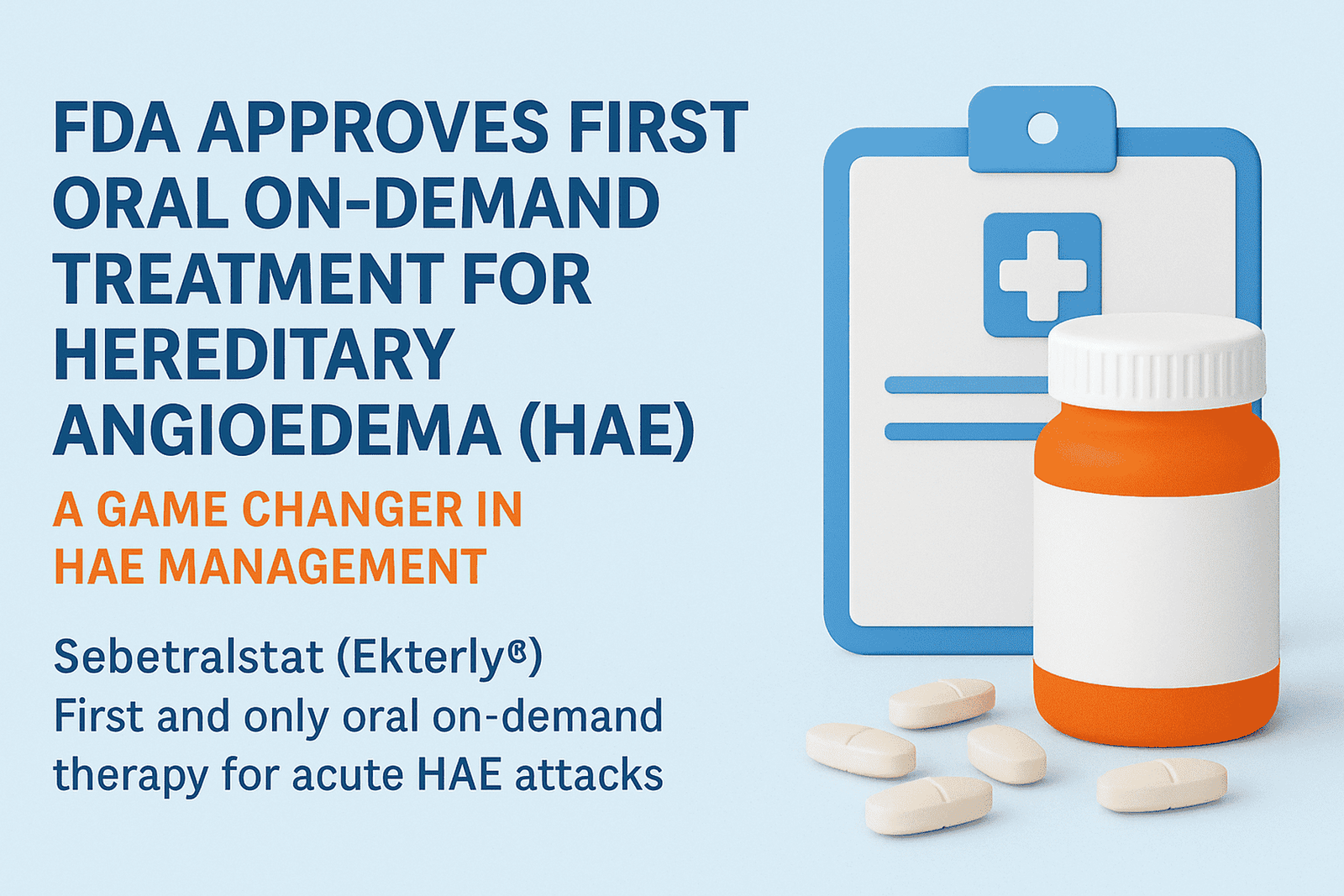
Read MoreSebetralstat: The First Oral Treatment for HAE Approved by FDA
Oral HAE treatment is now FDA-approved. On July 7, 2025, Sebetralstat (Ekterly®) became the first oral on-demand therapy for acute attacks in hereditary angioedema (HAE), marking a major shift in how this rare condition is managed. Hereditary Angioedema (HAE) is a rare genetic disorder that causes unpredictable, recurrent episodes of swelling, often affecting the face, throat, gastrointestinal tract, and extremities. If untreated, these attacks can be life-threatening. Until recently, on-demand treatment for HAE required injections only. While berotralstat (Orladeyo®) became the first oral medication approved for prophylaxis in 2020, acute…
Read MoreThe Basophil Activation Test (BAT): A Game-Changing Tool for Food Allergy Diagnosis
Diagnosing food allergies accurately remains one of the biggest challenges in allergy medicine. Traditional approaches like skin prick tests (SPT) and specific IgE blood tests can tell us if someone is sensitized to a food, but they don’t always predict whether that person will actually have an allergic reaction. Meanwhile, oral food challenges (OFCs)—the gold standard—are time-consuming, expensive, and carry real risks of severe reactions. Enter the Basophil Activation Test (BAT): a revolutionary diagnostic tool that’s changing how we approach food allergy diagnosis. 🧪 What Makes BAT Different? BAT is…
Read More